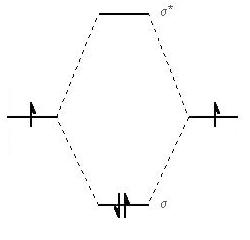
The simplest example of a σ bond is that formed between two hydrogen atoms.
First, let's consider what happens to the energy of two H atoms as they get closer to each other from infinite separation (see Figure 1.).
|
Energy (kJ/mol)
H2 Bond Dissociation Reaction
H - H bond distance (Å)
|
|
|||
The attraction of the electrons to the nuclei of the other atom stabilizes the system until they get so close that the nuclei start to repel each other. It is a balancing act between the attractive forces between nuclei and electrons, and replusions between nuclei and between electrons, see Figure 2 below.
The most stable (lowest energy) state corresponds to the equilibrium H-H bond length 74 pm.
The energy difference between the most stable state and the energy at infinite separation (zero by definition) corresponds to the H-H bond strength (or Bond Dissociation Energy (BDE)).
A of the formation of H2. (Note: the animation is large, wait until the background turns white before playing.)
A of the formation of H2, color coded by the electron density. (Again the animation is large, wait until the background turns white before playing.)
A of the formation of H2 σ* anti-bonding orbital. (Again the animation is large, wait until the background turns white before playing.)